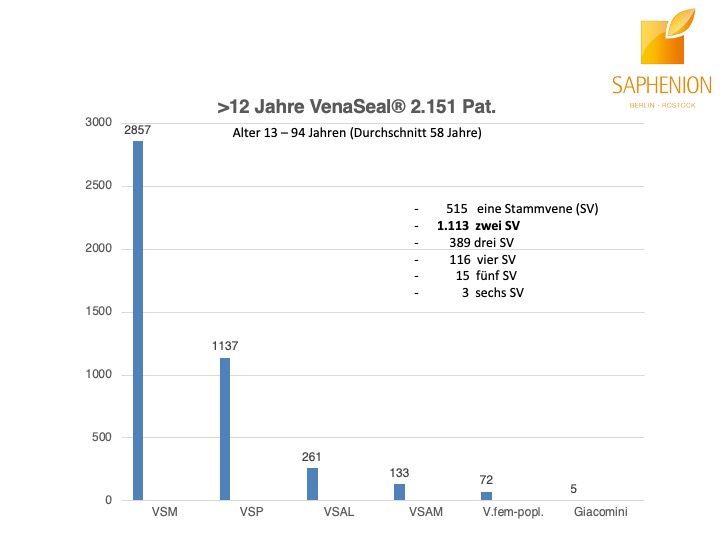
Saphenion®Rostock: Unser VenaSeal®-Vortrag – 12 Jahre Anwendung an 4465 Stammvenen
Die 40. Tagung der Deutschen Gesellschaft für Gefäßchirurgie fand in diesem Jahr vom 8. – 11. 10. 24 in Karlsruhe statt. Unser Team wurde zu einem Vortrag in der Sitzung zu neuen Therapie – Methoden bei der Behandlung von Krampfadern zugelassen und eingeladen.
Erstmals nahm auch Prof. Thomas Bürger an der Vorbereitung eines Vortrages vom Saphenion® – Team teil. Seine Leidenschaft und seine Ideen, gepaart mit den Erfahrungen aus seiner langen Zeit in der gefäßchirurgischen Wissenschaft, gaben uns ein paar neue Ideen und änderten auch ein wenig den wissenschaftlich – therapeutischen Blickwinkel. Dafür sei Prof. Bürger auch auf diesem Wege recht herzlich gedankt.
Saphenion®Rostock: Unser VenaSeal®-Vortrag





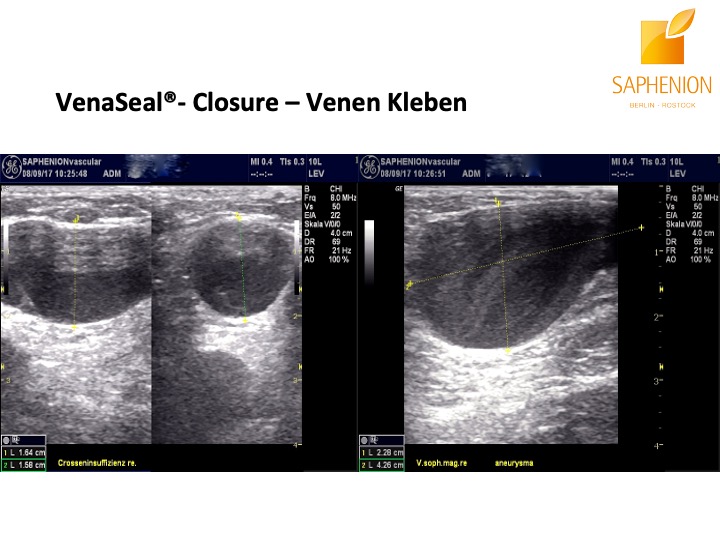
Saphenion®Rostock: Unser VenaSeal®-Vortrag: Sonographiebilder eines Crossenaneurysmas vor und nach der Therapie mit VenaSeal® Venenkleber


Saphenion®Rostock: Unser VenaSeal®-Vortrag – Fachliche Diskussion zu Vorteilen des Venenklebers




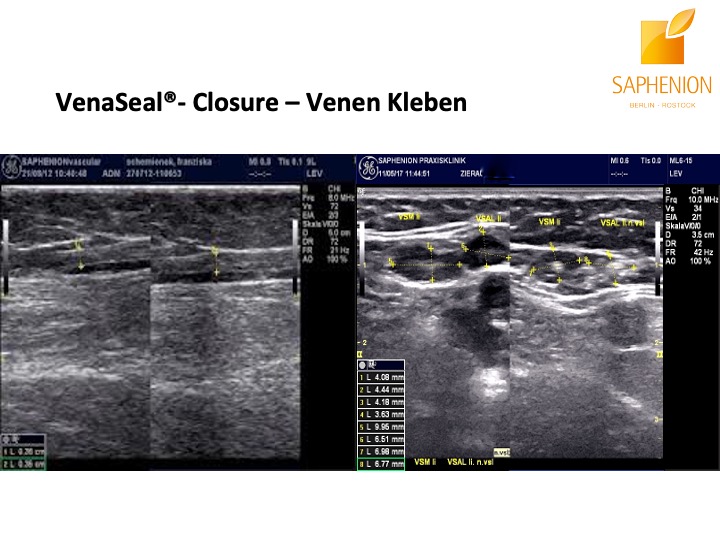
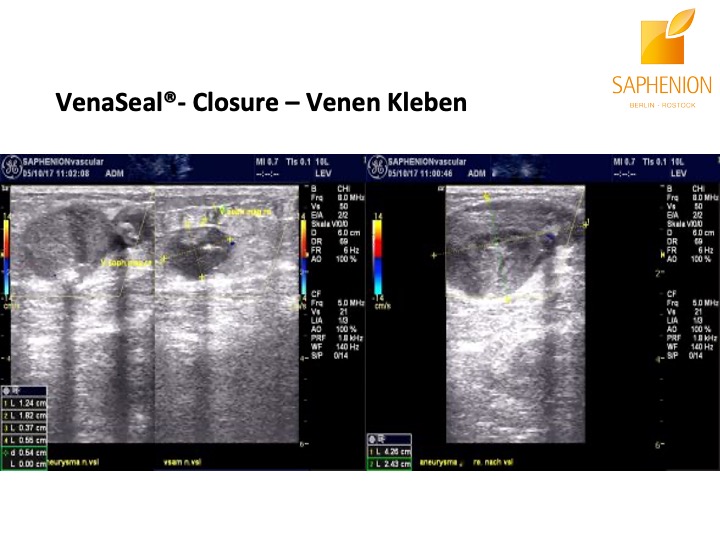
Saphenion®Rostock: Unser VenaSeal®-Vortrag: Sonographiebilder einer defekten Leistencrosse vor und nach der Therapie mit VenaSeal® Venenkleber.
Saphenion®Rostock: Unser VenaSeal®-Vortrag – Das Resümeè
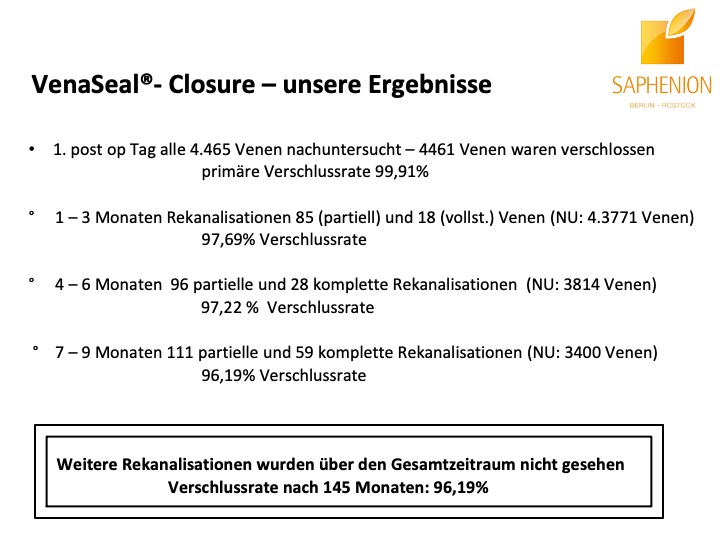
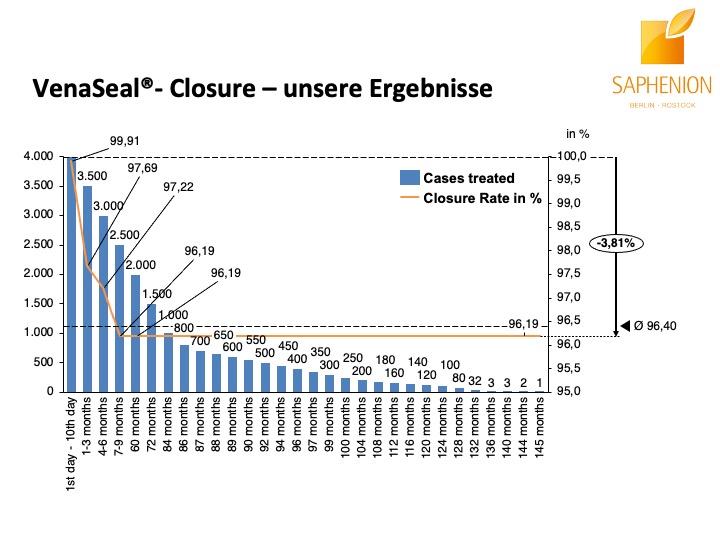
Nach unseren nunmehr über 12 Jahren Arbeit mit dem Venenkleber VenaSeal® lässt sich mit Fug und Recht sagen, daß diese Therapie – Methode die bisher eindeutig positivsten Ergebnisse und Resultate, wie auch fachlichen Erfahrungen und Patientenreaktionen gebracht hat. Wir führen diese Therapie nunmehr seit 145 Monaten durch, das ist ein Zeitraum, der nahezu der Hälfte unserer gesamten ambulanten Tätigkeit in der Gefäßmedizin entspricht.
Wir werden diese Methode trotz einigert weniger fachlicher Anfeindungen und und unsachlicher medialer Äußerungen nicht mehr verlassen und suchen weiterhin den engen Kontakt zu unseren Kollegen mit den gleichen sehr positiven Erfahrungen. Unser Ziel wird es auch in der Zukunft bleiben, das Spektrum und die Indikationen für diese Methode zu erweitern und zu präzisieren. Es wäre sehr schön, wenn auch die Gesetzlichen Krankenkassen sich dieses Themas annehmen würden.
Und am Schluß noch einmal ein grosses Dankeschön an unseren Professor Bürger – er bestärkt uns regelmäßig in unseren Ansichten zu diesem Therapieverfahren, er erlebt diese Methode nunmehr seit 18 Monaten in unserer Praxis und hat sich als Vorstand der Leitlinienkomission der DGG inzwischen fachlich gefäßchirurgisch eindeutig positiv zur VenaSeal® – Venenkleber – Therapie bekannt!
Günther Fischer und Band und als Gast Uschi Brüning.
PHOTOS VIDEO: UTZIUS
LINKS / LITERATURE:
Kopie von Seite 11 des FDA – Approvals zur Biokompatibilität des VenaSeal® – Venenklebers:
Table 4: Results of Biocompatibility Testing – VenaSeal Adhesive (Polymerized and the Unpolymerized States) Test Method Reference Results Cytotoxicity (Elution Method) ISO 10993-5.
The cumulative results of the VenaSeal adhesive material cytotoxicity testing, in combination with assessments of toxicological risk and in vivo use, support an overall favorable cytotoxicity profile for the VenaSeal adhesive material per its intended use.
ISO MaximizationSensitization Study(Guinea Pigs)ISO 10993-10VenaSeal adhesive does not elicit a sensitization response
ISO Intracutaneous Reactivity – ISO 10993-10: The cumulative results support that the VenaSeal® adhesive material does not cause intracutaneous reactivity (Material Mediated Rabbit Pyrogenicity
ISO 10993-5US Pharmacopeia Section 151: The cumulative results support that the VenaSeal adhesive material is non-pyrogenic.
Acute Systemic Toxicity- ISO 10993-11: The cumulative results support that the VenaSeal adhesive material is not considered to cause acute systemic toxicity
Subacute / Subchronic ToxicityImplantation (13 weeks) – ISO 10993-11 / ISO 10993-6: The cumulative results support that the VenaSeal adhesive material does not result in any specific adverse systemic toxicological findings in the tissues examined Genotoxicity (Bacterial Mutagenicity, invitro Mouse Lymphoma Assay, Mouse Micronucleus Assay)
ISO 10993-3: The cumulative results support that the VenaSeal adhesive material is non-mutagenic Hemo-compatibility (Hemolysis, Complement Activation, Partial Thromboplastin Time, Platelet and Leukocyte Count), ASTM F-756-08
ISO 10993-4: The cumulative results support that the VenaSeal adhesive material is non-hemolytic and not chronic toxicity Implantation (26 Weeks)
ISO 10993-11/ ISO 10993-6: The cumulative results support that VenaSeal – adhesive does not cause any significant adverse systemic or local toxicity in the tissues examined.
Quelle: Summary – Food and Drug Administration / FDA
Almeida JI, Murray SP, Romero ME. Saphenous vein histopathology 5.5 years after cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2020 Mar;8(2):280-284. doi: 10.1016/j.jvsv.2019.04.014. Epub 2019 Jul 4. PubMed PMID: 31281102.
Bozkurt AK, Balkanay OO, Dinc R. Comparative analysis of VenaBlock and VenaSeal Systems for catheter-guided endovenous cyanoacrylate closure in treating chronic venous insufficiency of the lower extremity: effectiveness and feasibility. Int Angiol. 2024 Jun;43(3):331-341. doi: 10.23736/S0392-9590.24.05143-5. Epub 2024 Jul 23. PMID: 39041783.
Chan YC, Law Y, Cheung GC, Ting AC, Cheng SW. Cyanoacrylate glue used to treat great saphenous reflux: Measures of the outcome. Phlebology. 2017 Mar;32(2):99-106. doi 10.1177/0268355516638200. Epub 2016 Jul 9. PubMed PMID: 27052039.
Chan SSJ, Yap CJQ, Tan SG, Choke ETC, Chong TT, Tang TY. The utility of endovenous cyanoacrylate glue ablation for incompetent saphenous veins in the setting of venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020 Mar 20. PII: S2213-333X(20)30100-1. doi: 10.1016/j.jvsv.2020.01.013. [Epub ahead of print] PubMed PMID: 32205130.
Cho S, Park HS, Lee T, Byun SJ, Yun WS, Yang SS, Kim H, Kim WS, Joh JH, Jung IM. CASS (CyanoAcrylate closure versus Surgical Stripping for incompetent saphenous veins) study is a randomized controlled trial comparing clinical outcomes after cyanoacrylate closure and surgical stripping for the treatment of incompetent saphenous veins. Trials. 2020 Jun 3;21(1):460. doi 10.1186/s13063-020-04393-0. PMID: 32493398; PMCID: PMC7268719.
Comby PO, Guillen K, Chevallier O, Lenfant M, Pellegrinelli J, Falvo N, Midulla M, Loffroy R. Endovascular Use of Cyanoacrylate-Lipiodol Mixture for Peripheral Embolization: Properties, Techniques, Pitfalls, and Applications. J Clin Med. 2021 Sep 23;10(19):4320. doi: 10.3390/jcm10194320. PMID: 34640339; PMCID: PMC8509239.
García-Carpintero E, Carmona M, Chalco-Orrego JP, González-Enríquez J, Imaz-Iglesia I. Systematic review and meta-analysis of endovenous cyanoacrylate adhesive ablation for incompetent saphenous veins. J Vasc Surg Venous Lymphat Disord. 2020 Mar;8(2):287-296. doi: 10.1016/j.jvsv.2019.09.010. Epub 2020 Jan 6. PMID: 31917181.
Gibson K, Ferris B. Cyanoacrylate closure of incompetent great, small, and accessory saphenous veins without post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular. 2017 Apr;25(2):149-156. doi 10.1177/1708538116651014. Epub 2016 Jul 9. PubMed PMID: 27206470.
Gibson K, Morrison N, Kolluri R, Vasquez M, Weiss R, Cher D, Madsen M, Jones A. Twenty-four month results from a randomized trial of cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2018 Sep;6(5):606-613. doi: 10.1016/j.jvsv.2018.04.009. Epub 2018 Jun 15. PubMed PMID: 29914814.
Jones AD, Boyle EM, Woltjer R, Jundt JP, Williams AN.J Vasc Surg Cases Innov Tech. 2019 Aug 7;5(3):372-374. doi: 10.1016/j.jvscit.2019.05.004. eCollection 2019 Sep.PMID: 31440717
Jones AD, Boyle EM, Woltjer R, Jundt JP, Williams AN. Persistent type IV hypersensitivity after cyanoacrylate closure of the great saphenous vein. J Vasc Surg Cases Innov Tech. 2019 Aug 7;5(3):372-374. doi: 10.1016/j.jvscit.2019.05.004. eCollection 2019 Sep. PubMed PMID: 31440717; PubMed Central PMCID: PMC6699189.
Kiguchi MM, Reynolds KB, Cutler B, Tefera E, Kochubey M, Dirks R, Abramowitz SD, Woo EY, O’Banion LA. Perforator treatment is needed after VenaSeal and ClosureFast endovenous saphenous vein closure in CEAP 6 patients. J Vasc Surg Venous Lymphat Disord. 2021 Jun 7:S2213-333X(21)00295-X. doi: 10.1016/j.jvsv.2021.04.020. Epub ahead of print. PMID: 34111593.
Kolluri R, Chung J, Kim S, Nath N, Bhalla BB, Jain T, Zygmunt J, Davies A. Network meta-analysis to compare VenaSeal with other superficial venous therapies for chronic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2020 Feb 13. PII: S2213-333X(19)30702-4. doi: 10.1016/j.jvsv.2019.12.061. [Epub ahead of print] Review. PubMed PMID: 32063522.
Nasser H, Ivanics T, Shakaroun D, Lin J. Severe phlebitis-like abnormal reaction following great saphenous vein cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2019 Jul;7(4):578-582. doi: 10.1016/j.jvsv.2019.03.010. Epub 2019 May 8. PubMed PMID: 31078516.
Navarro-Triviño FJ, Cuenca-Manteca J, Ruiz-Villaverde R. Allergic contact dermatitis with systemic symptoms caused by VenaSeal. Contact Dermatitis. 2020 Mar;82(3):185-187. doi 10.1111/cod.13431. Epub 2019 Nov 15. PubMed PMID: 31674037.
Park I. Successful use of VenaSeal system for the treatment of large great saphenous vein of 2.84 cm diameter. Ann Surg Treat Res. 2018 Apr;94(4):219-221. doi: 10.4174/astr.2018.94.4.219. Epub 2018 Mar 26. PubMed PMID: 29629358; PubMed Central PMCID: PMC5880981.
Park I. Initial Outcomes of Cyanoacrylate Closure, VenaSeal System, for treating the Incompetent Great and Small Saphenous Veins. Vasc Endovascular Surg. 2017 Nov;51(8):545-549. doi10.1177/1538574417729272. Epub 2017 Oct 2. PubMed PMID: 28969499.
Park I, Kim D. Correlation Between the Immediate Remnant Stump Length and Vein Diameter After Cyanoacrylate Closure Using the VenaSeal System During Treatment of an Incompetent Great Saphenous Vein. Vasc Endovascular Surg. 2019 Oct 3:1538574419879563. doi:10.1177/1538574419879563. [Epub ahead of print] PubMed PMID: 31581906.
Shaĭdakov EV, Mel’tsova AZ, Porembskaia OI, Kudinova EA, Korzhevskiĭ DÉ, Kirik OV, Sukhorukova EG. [Experience with using cyanoacrylate glue in endovascular treatment of varicose veins]. Angiol Sosud Khir. 2017;23(4):62-67. Russian. PubMed PMID: 29240057.
Lam YL, De Maeseneer M, Lawson J, De Borst GJ, Boersma D. Expert review on the VenaSeal® system for endovenous cyanoacrylate adhesive ablation of incompetent saphenous trunks in patients with varicose veins. Expert Rev Med Devices. 2017 Oct;14(10):755-762. doi 10.1080/17434440.2017.1378093. Review. PubMed PMID: 28892412.
Lane TR, Kelleher D, Moore HM, Franklin IJ, Davies AH. Cyanoacrylate glue for the treatment of great saphenous vein incompetence in the anticoagulated patient. J Vasc Surg Venous Lymphat Disord. 2013 Jul;1(3):298-300. doi: 10.1016/j.jvsv.2012.09.007. Epub 2013 Feb 15. PubMed PMID: 26992590.
Morrison N, Gibson K, McEnroe S, Goldman M, King T, Weiss R, Cher D, Jones A. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. 2015 Apr;61(4):985-94. doi: 10.1016/j.jvs.2014.11.071. Epub 2015 Jan 31. PubMed PMID: 25650040.
Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2020 Mar 20. PII: S2213-333X(20)30105-0. doi: 10.1016/j.jvsv.2019.12.080. [Epub ahead of print] PubMed PMID: 32205125.
O’Banion LA, Reynolds KB, Kochubey M, Cutler B, Tefera EA, Dirks R, Kiguchi MM. Treatment of superficial venous reflux in CEAP 6 patients: a comparison of cyanoacrylate glue and radiofrequency ablation techniques. J Vasc Surg Venous Lymphat Disord. 2021 Jan 13:S2213-333X(21)00001-9. doi: 10.1016/j.jvsv.2020.12.082. Epub ahead of print. PMID: 33453440.
Park I. Human Saphenous Vein Histopathology 2 Years After Cyanoacrylate Closure Using the VenaSeal™ System. Ann Vasc Surg. 2021 Feb;71:534.e17-534.e21. doi: 10.1016/j.avsg.2020.09.017. Epub 2020 Sep 16. PMID: 32949737.
Tang TY, Yap CJQ, Chan SL, Soon SXY, Yap HY, Lee SQW, Choke ETC, Chong TT. Early results of an Asian prospective multicenter VenaSeal real-world postmarket evaluation to investigate the efficacy and safety of cyanoacrylate endovenous ablation for varicose veins. J Vasc Surg Venous Lymphat Disord. 2021 Mar;9(2):335-345.e2. doi: 10.1016/j.jvsv.2020.03.020. Epub 2020 May 7. PMID: 32387378.
Watts TJ, Thursfield D, Haque R. Allergic contact dermatitis caused by VenaSeal tissue adhesive. Contact Dermatitis. 2019 Jun;80(6):393-395. doi 10.1111/cod.13206. Epub 2019 Jan 30. PubMed PMID: 30582174.
Wilczko J, Szary C, Plucinska D, Grzela T. Two-Year Follow-Up after Endovenous Closure with Short-Chain Cyanoacrylate versus Laser Ablation in Venous Insufficiency. J Clin Med. 2021 Feb 7;10(4):628. doi: 10.3390/jcm10040628. PMID: 33562190; PMCID: PMC7914451.
https://www.ajsccr.org/index.php
Zierau UT and Lahl W: Recurrence Discussion in Varicose Veins Therapy – A Critical Examination of the Vein Stump discussion; J. Vasc. Endovasc. Therapy 2019, Vol.4 No.2:13
